Biopharma News Week1-2017

Author: Jean-Claude Muller, 穆卓Executive Editor at BtoBioInnovation, jcm@btobioin
Editor’s Note
We wish all our subscribers and readers a Happy and Successful Year 2017
The year 2016 finished with an impressive scientific triumph that will change the way the medical community will fight the Ebola virus. An experimental Ebola vaccine named rVSV-ZEBOV, tested on humans in a major trial in Guinea, is not only the first vaccine to protect from the terrifying Ebola virus, but it has also induced 100% protection against infection from one of the most lethal known pathogens.
The year 2016 will also be remembered by the impressive number of clinical development setbacks, a low number of new drug approvals and an scary M&A valuation of biopharmaceutical companies. Expectations for 2017 are hopefully deemed to overcome the poor performance achieved by the entire biopharmaceutical industry in 2016.
Acquisitions /mergers/joint-ventures
Sanofi announces the completion of two divestures as of January 1, 2017. The exchange of Merial, its animal health business, with Boehringer Ingelheim’s consumer health care and the end of Sanofi Pasteur MSD, its vaccine joint venture with Merck.
Business
Servier (Suresnes, France):
enters a worldwide license option agreement with OSE Immunotherapeutics (Nantes, France) to develop and commercialize Effi-7, a monoclonal immunomodulatory antibody targeting the alpha chain of the interleukin-7 receptor (IL-7R-alpha or CD127 receptor), for the treatment of unmet needs in auto-immune diseases. Under the terms of the deal Servier will make an upfront payment of €10.25 million and additional payments of €30 million upon the exercise of a two-steps option license. Further payments of up to €242 million will be linked to the achievement of development, registration and sales milestones in various indications as well as double-digit royalties on sales.
…and partners with Pieris Pharmaceuticals (Boston, MA, USA) to develop Pieris’ preclinical dual-checkpoint inhibitor PRS-332 and up to seven other immune-oncology bispecific candidates in a total deal worth up to $1.8 billion. In the initial phase both partners will pursue five bispecific programs led by preclinical candidate PRS-332 a programmed cell death protein (PD-1)- targeting bispecific checkpoint inhibitors. Pieris' multispecific technology allows simultaneous checkpoint inhibition on the same cell, which could have a clear advantage over monoclonal antibody combination against different checkpoint targets. Pieris and Servier will jointly develop PRS-332 and split commercial rights geographically, with Pieris retaining all U.S. commercial rights and Servier having commercial rights elsewhere in the world.
Novartis enters an exlcusive global option and collaboration deal with Ionis Pharmaceuticals (Carlsbad, CA, USA) a fully owned subsidiary of Akcea Therapeutics (Cambridge, MA, USA) to develop and commercialize AKCEA-APO(a)-Lrx and AKCEA-APOIII-Lrx, two antisense drug candidates to treat lipid disorders in a deal valued at $1.7 billion. Lp(a) is a lipoprotein particle that is associated with an increased risk of coronary heart disease. ApoC-III is a protein produced in the liver that regulates serum triglycerides. Under the terms of the agreement, Novartis will make a $75M upfront option payment, a $150M license fee for each drug, up to $315M in development and regulatory milestones for AKCEA-APO(a)Lrx, up to $265M in regulatory and development milestones for AKCEA-APOIII-Lrx and up to $285M and $265M, respectively, in commercial milestones. Novartis will also will invest $100M in Ionis via the purchase of common shares and make an additional $50M investment in the next 18 months either in Ionis or Akcea.
Otsuka Pharmaceutical (Tokyo, Japan) enters a co-development deal with Akebia Therapeutics (Cambridge, MA, USA) for vadadustat in the US as a treatment for chronic disease-related anemia. Vadadustat inhibits hypoxia inducible factor-prolyl hydroxylase (HIP-PH) leading to stabilization and increased levels of HIFα. There are currently no approved therapies that utilize this mechanism of action. Under the terms of the deal Otsuka will commit a $265 million payment and additional milestones totaling $765 million. Akebia already has a $350 million agreement in place with Mitsubishi Tanabe for the Asian markets, and has been searching for a European partner since 2015 where it has been locked in patent disputes with FibroGen.
Sanofi elects to continue its partnesrship with MyoKardia (South San Francisco, CA, USA) and makes a $45 million milestone payment. The collaboration includes three MyoKardia programs, two in hypertrophic cardiomyopathy (HCM) and one in dilated cardiomyopathy (DCM). MYK-461, currently in phase 2, is being developed to treat symptomatic obstructive HCM, an Orphan Drug designation by the US FDA. MYK-491, a DCM candidate, should enter Phase 1 clinical trial in early 2017.
Tiziana Life Sciences (London, UK) in-licenses NI-1201, a fully human anti-interleukin-6 monoclonal antibody from Novimmune (Basel, Switzerland) for an undisclosed amount. Tiziana indicates that in contrast with other anti-IL-6R mAbs, such as Roche’s Actemra®(tocilizumab), which exert their effects predominantly through binding to a membrane-bound receptor, NI-1201 acts both on membrane-bound IL-6R and on soluble IL-6R and is also able to deplete IL-6 circulating in blood. Tiziana believes that the unique mechanism of action of NI-1201 could also complement its own fully human oral anti-CD3 mAb candidate foralumab (NI-0401).
Johnson & Johnson has negotiated a license to acquire Bird Rock Bio (La Jolla, CA, USA) and its main asset mamacizumab, an experimental cannabinoid 1 receptor antibody for the treatment of non-alcoholic steatohepatic disease (NASH) and possibly diabetic kidney disease. According to Bird Rock Bio mamacizumab is the only CB1 program for NASH in the clinic. Financial terms of the deal were not disclosed.
Shire spins out its mRNA activity, including its staff, to RaNA Therapeutics (Cambridge, MA, USA) in exchange for undisclosed equity.
Special Report
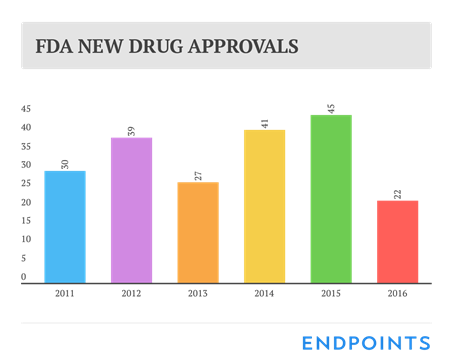
According to a report by Novasecta (London, UK) the value of the buyout of biopharmaceutical companies is getting heavily inflated with a median value of 39 times revenue in 2016, compared to 19 times revenues in 2015 and 8 times in 2014. This comes in sharp contrast with a very high rate of major clinical development failures and setbacks all over the industry. In one of his recent newsletter John Caroll states “2016 has to rank as one of the worst years in recent drug R&D history. We have been treated to everything from repeat Phase 3 debacle in Alzheimer’s, to back to back clinical holds on a CAR-T drug, we have seen stellar organization R&D team overstep while some went from doing no wrong to doing no right… including a series of biotech disasters…” From a business investor’s point of view, 2016 looks like a worst-case scenario with a lineup of notable setbacks in almost all the companies’ pipelines and an historical low number of 22 newly approved drugs by the US FDA. There is therefore no coincidence that the Return on Investment (ROI) has continued to slide to as low as 3.7% in 2015, according to Deloitte’s analyst (see newsletter #2016.50) and should continue to do so in 2016, in spite of steadily increasing pricing of new drugs. On top of the overall poor performance, we see that M&A inflation has pushed the biopharmaceutical valuation to a scary high peak when ROI is at it’s lowest level ever. Any resemblance with the “Internet Bubble” in the year 2000 sounds more than just coincidence. It would not be surprising if this were a topic of importance at the JP Morgan 35thAnnual Healthcare Conference next week in San Francisco.
Approval of drugs and vaccines
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has recommended 81 new medicine in 2016, a drop from 2015's tally of 93, including seven at its December meeting.
The US FDA has approved 22 new drugs (9 biologicals) in 2016, 20 drugs approved by CDER and 2 rDNA drugs approved by CBER. Similar add-up for 2015 was 45. The 13 top Big Pharma got 9 approvals in 2016 versus 21 in 2015. Roche, Merck and Lilly got 2. Sanofi, Gilead and Pfizer got one. Johnson & Johnson, GlaxoSmithKline, Novartis, Amgen, Bristol-Myers Squibb, AstraZeneca and Bayer got none. Biogen got 2 with two different partners. Percentage of first in class was 41% the same figure as in 2015. The US FDA has also approved four biosimilars, but launching remains an issue partly because of ongoing litigations on patent infringements.
US FDA has published its final guidance entitled “Clinical Pharmacology Data to Support a Demonstrationof Biosimilarity to a Reference Product” to aid biosmilar drug producers with the design of the studies requested to support a registration.
Drug at clinical stage
US FDA grants orphan status to:
Marinus Pharmaceuticals’ (Radnor, PA, USA) ganaxolone, a synthetic analog of allopregnanolone a GABA modulator, as a treatment for fragile X syndrome, the most common genetic cause of autism. The drug already has similar status for the treatment of status epilepticus, an emergency situation form of epilepsy characterized by prolonged or repeated grand mal seizures as well as for the treatment of patients with protocadherin 19 gene family epilepsy, a rare syndrome affecting young females.
US FDA grants fast track status to:
Fibrocell Sciences (Exton, PA, USA) FCX-007, a gene therapy product as a treatment for recessive dystrophic epidermolysis bullosa. FCX-007 is an autologous fibroblast that encodes the gene for protein VII collagen. Recessive Dystrophic Epidermolysis Bullosa is a rare, congenital, debilitating genetic skin disorder.
…and to Neurelis’ (San Diego, CA, USA) intranasal diazepam NRL-1 as a treatment for acute repetitive or cluster seizures for patients with refractory epilepsy on a stable regimen of anti-epilpetic drugs. Intranasal NRL-1 already received orphan status from the US FDA.
Medical Devices and diagnostis News
Johnson & Johnson has unveiled a series of new collaborations including 3D printing of an artificial meniscus and creating a personalized sleep coaching system for babies:
with Aspect Biosystems (Vancouver, Canada), a company specializing in 3D printing and tissue engineering to create a protype artificial meniscus using its Lab-on-a Printer 3D technology
with Rest Devices (Boston, MA, USA) to create Nod, an application to help parents ensure their babies sleep more soundly
In a world where medical devices with more interconnectivity there is also an increasing risk of cybersecurity threats. The US FDA has published its final guidance on the postmarket management of cybersecurity in medical devices last week. The recommendations apply to medical devices that use software, including programmable logic and software that is regulated as a medical device, including mobile medical apps.
Science and technology
An experimental Ebola vaccine, named rVSV-ZEBOV was highly protective against the deadly virus in a major trial in Guinea, according to results of a new study reported by The Lancet. Since Ebola was discovered in the former Zaire in 1976, there have been many efforts to create a vaccine. Although only about 1,600 people died of Ebola over those years, the nature of their deaths — copious hemorrhaging from every orifice — has lent the disease a frightening reputation. The many small Ebola outbreaks that occurred between 1976 and 2014 were all stopped in remote villages by laborious methods: medical teams flew in, isolated the sick, and donned protective gear to treat them and bury the dead. But that tactic failed in 2014 when the virus reached crowded capital cities and took 11,000 lives in Africa and a few people in Europe and the United States. The new vaccine, developed over a decade ago by the Public Health Agency of Canada and the United States Army, now licensed to Merck, was not ready in time to stop the outbreak, which probably began in a hollow, bat-filled tree in Guinea and swept Liberia and Sierra Leone. The Ebola trial was led by the World Health Organization, the Guinean Health Ministry, Norway’s Institute of Public Health and other institutions. The reported study was done in 11,841 residents of Guinea last year. Among the 5,837 people who got the vaccine, none came down with Ebola 10 or more days later. There were 23 Ebola cases among the thousands of others not immediately vaccinated.
Cost, Pricing and Market Access
Biogen and partner Ionis Pharameceuticals have priced Spinraza (nusinersen), their newly approved pioneering gene therapy product for rare cases of spinal muscular atrophy at $125,000 per injection which adds up to about $750,000 for the first year and $350,000 each year after, or $1.5 million over three years. A major drug price controversy may soon erupt on this shocking pricing for a rare disease.
The UK National Institute for Health and Care Excellence (NICE) supports the use of Amicus Therapeutics’ (Cranbury, NJ, USA) Galafold for treating Fabry disease, a rare genetic disease caused by a non-functional or only partially functional enzyme called alpha-galactosidase A (alpha-gal A), which results in the build up of enzyme substrates that cause cellular damage in tissues throughout the body. Galafold, or migalastat, is an oral, small molecule drug which binds to this enzyme, helping it to fold correctly and improving its function. The drug was approved in Europe in May 2016 with a price tag in the UK of £210,000 per patient per year.
According to a QuintilesIMS Institute report the global drug spending is expected to reach $1.5 trillion by 2021, up 33% from 2016 with historical numbers of first in class medicines emerging from the industry pipelines. The report indicates that medicine spending will grow at 4% to 7% compound annual rate during the next five years down from the approximate 9% growth in 2014 and 2015 which were driven by one-time anomalities in the US.
Miscellaneous
Sanofi and Regeneron Pharmaceuticals lose their bid to overturn a court verdict upholding two Amgen patents covering cholesterol drug Repatha. Federal Judge Sue Robinson has now decided to block Sanofi and Regeneron from selling Praluent in the U.S. and has ordered that the ban not take effect for 30 days to give Sanofi and Regeneron time to appeal. Both defendants have indicated that tey will appeal to the U.S. Federal Circuit Court of Appeals, which reviews patent disputes. "It is our longstanding position that Amgen's patent claims are invalid and that the best interests of patients will be greatly disserved by an injunction preventing access to Praluent," Sanofi's Executive Vice President of Legal Affairs and General Counsel Karen Linehan said in a statement.
People on the move:
David Epstein, former Novartis pharmaceutical chief is joining the VC Flagship Pioneering (Cambridge, MA, USA) as an executive partner and will be appointed chairman at Rubius Therapeutics.
Allesandro Riva, former oncology head at Novartis joins Gilead to head its cancer team.
Bioevents
Pharma & Biotech events in 2017
Invitro Diagnostic events in 2017
9th Biotech Showcase Investor Conference on January 9-11, in San Francisco (USA)
JP Morgan 35th Annual HealthCare Conference on January 9-12, in San Francisco (USA)
BIO CEO & Investor Conference on February 13-17, New York City (USA)
European Life Sciences CEO Forum & Exhibition on March 6-7, Zurich (Switzerland)
BIO Asia on March 14-15, in Tokyo (Japan)
BIO-Europe Spring on March 20-22, in Barcelona (Spain)
9th Annual China BIO Partnering Forum on May 10-11 in Shunde (China)
BIO International Convention on June 19-22 in San Diego (USA)
BIOPharm America on September 26-27 in Boston (USA)
BIO Latin America on October 26-28 in Sao Paulo (Brazil)
BIO Europe on Novmber 6-8 in Berlin (Germany)
BIOFIT on November 28-29 in Strasbourg (France)
This document has been prepared by btobioinnovation and is provided to you for information purposes only. The information contained in this document has been obtained from sources that btobioinnovation believes are reliable but btobioinnovation does not warrant that it is accurate or complete. The views presented in this document are those of btobioinnovation’s editor at the time of writing and are subject to change. btobioinnovation has no obligation to update its opinions or the information in this document.
![]()
Discover our services in Marketing & Business Development:
Last News
- Alzheimer’s disease : Beyond the beta amyloid hypothesis
- Un monde posteuropéen : déjà une réalité ?
- PIB, Budget, Dépenses : Une mise au point.
Events
News archives
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- March 2024
- February 2024
- January 2024
- November 2023
- September 2023
- July 2023
- April 2023
- March 2023
- January 2023
- December 2022
- November 2022
- October 2022
- August 2022
- June 2022
- May 2022
- April 2022
- March 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- October 2018
- June 2018
- May 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- September 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- November 2013
- September 2013
- July 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- March 2012







