Biopharmaceutical News Week 31
Acquisitions /mergers/joint-ventures
Teva acquires Allergan for $40.5 billion and forget everything you have been reading over the last months about the Teva-Mylan deal. Now the Israeli-based company has announced its intention to acquire Allergan’s generic business through a deal structured around $33.75 billion in cash and shares valued at $6.75 billion giving Allergan a 10% stake in Teva. Mylan congratulated Teva on the deal and reiterated its commitment to acquire Perrigo.
…..And in the meantime Allergan acquires Naurex (Evanston, IL, USA) for $650 million which gives it access to two experimental NMDA modulators, raspastinel (GLYX-13) and NRX-1074 for the treatment of depression. Under the terms of the deal, Allergan will pay $460 million upon the closing of the acquisition and $100 million by January next year as well as potential success and sales milestone payments.
Merck acquires Israel-based biotech cCAM for $605 million and extends its immuno-oncology portfolio. The deal is structured around a $95 million upfront to acquire all outstanding cCAM stocks and up to $510 million in milestone payments.
TiGenix (Belgium) acquires Coretherapix (Spain) and boosts its cardiology pipeline with the access to AlloCSC-01 an allogeneic cardiac stem cell product for the treatment of chronic ventricular tachycardia. Under the terms of the deal Tigenix will pay an upfront of €1.2 million in cash and €5.5 million in new Tigenix shares as well as another €15 million in Tigenix shares upon successful clinical milestones and up to $245 million in sales milestones.
Business
Sanofi Genzyme strengthens its endocrinology portfolio in acquiring Caprelsa (vandetanib) from AstraZeneca for the treatment of symptomatic or progressive medullary thyroid carcinoma, a rare disease. Under the terms of the agreement, Sanofi Genzyme will pay AstraZeneca up to $300 million, including an upfront payment of $165 million and further development and sales milestones payments of up to $135 million.
….And Sanofi extends its collaboration with Regeneron and enters the immunotherapy field. The initial term of the partnership is five years. Companies agree to jointly advance PD-1 and other new immuno-oncology antibodies. Sanofi commits to an initial investment of up to $2.17 billion in the exclusive collaboration, including $640 million in upfront payments to Regeneron and a potential sales milestone of $375 million.
Laboratoires Guerbet (France) acquires Mallinckrodt’s contrast product business for $270 million and increases its international market share, particularly in the US.
Novartis spins out three of its mid-to late-stage biological products to UK-based Mereo Biopharma, namely: BPS-804 for the treatment of brittle bone syndrome, BCT-197 for acute exacerbations in chronic obstructive pulmonary disease (COPD) and BGS-649 to normalize testosterone levels in men with hypogonadotrophic hypogonadism. Under the terms of the deal Novartis will receive milestones and royalties on commercial sales.
Approval of drugs, vaccines, diagnostics and devices
US FDA approves Sanofi and Regeneron’s Praluent (alirocumab), the first PCSK9 inhibitor available in the United States, a treatment of high LDL cholesterol in adults, to be injected once-every-two-weeks. The U.S. Wholesale Acquisition Cost (WAC) price of Praluent is $40 per day ($1,120 every 28 days) for both the 75 mg and 150 mg doses. Express Scripts takes a dim view on the proposed price which is several orders of magnitude above the costs of statins which average $2-3 per day.
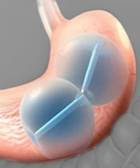
US FDA approves ReShape Integrated dual balloon system, a minimally invasive option for obese patients. The device consists of two salt water-filled silicon balloons that reduce appetite by taking up space in the stomach "or by other mechanisms that are not yet fully understood," according to the FDA.
ReShape Integrated Dual Balloon System–Courtesy of ReShape Medical
Drugs at clinical stage
US FDA has awarded breakthrough therapy status to Progenics Pharmaceuticals' Azedra, a treatment for patients with pheochromocytoma and paraganglioma, both rare neuroendocrine tumors.
Miscellaneous
A two-step regimen of an experimental MERS vaccine showed promising results in animal studies.
Merck's Ebola vaccine, in development alongside NewLink Genetics, protected 100% of patients from contracting the virus in interim results from an ongoing phase 3 study conducted in Guinea.
Romanian authorities are investigating 11 pharma companies under suspicion of bribing physician to prescribe cancer drugs. In a statement, the Romanian National Anti-corruption Directorate (DNA) said: "The file is related to suspicions of corruption offences regarding the manner in which a series of medicines were prescribed and purchased by certain health institutions and medical doctors in Romania.”
Bioevents
- Pharmaceutical Congress Asia 2025 on August 3-6 in Singapore
- 3rd Annual Nordic Life Science Days Partnering Conference on September 9-10 in Stockholm (Sweden)
- BioPharm America 2015 on September 15-17 in Boston (USA)
- Innovation Days on October 5-6 in Paris (France)
- BIO Japan on October 13-16 in Yokohama (Japan)
- BIO Latin America on October 14-16 in Rio de Janeiro (Brazil).
- 15th Annual Biotech in Europe Forum for Global Partnering & Investment on September 29-30 in Basel (Switzerland)
- BIO Europe 2015 on November 2-4 in Munich (Germany)
- Biofit 2015 on December 1-2 in Strasbourg (France)
Author: Jean-Claude Muller, Special Advisor at I&IR, jcm@btobioinnovation.com
Discover our services in Marketing & Business Development:
See All News
See other Biopharmaceutical News
see other Pharma & Biotech events in 2015
Last News
- Eli Lilly wins FDA approval for donanamab
- Le système des retraites en France
- FDA Advisory Committee recommends approval of Eli Lilly donanemab
Events
News archives
- July 2024
- June 2024
- March 2024
- February 2024
- January 2024
- November 2023
- September 2023
- July 2023
- April 2023
- March 2023
- January 2023
- December 2022
- November 2022
- October 2022
- August 2022
- June 2022
- May 2022
- April 2022
- March 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- October 2018
- June 2018
- May 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- September 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- November 2013
- September 2013
- July 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- March 2012